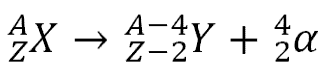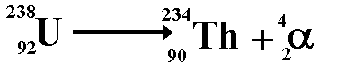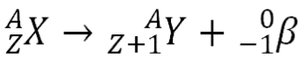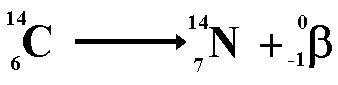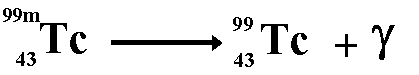P15. Radioactivity
Radioactivity
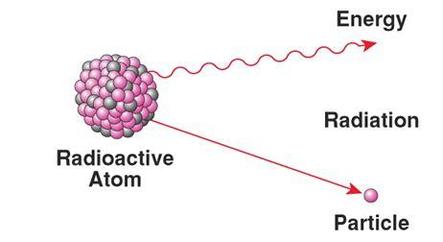
Elements having an atomic number greater than 84 such as plutonium (Z = 94), Radium (Z = 88), Uranium (Z = 92) etc. are unstable by nature. These elements continuously emit different types of powerful radiations. This phenomenon is known as radioactivity.
As a result of emission of radiation the atoms of these elements undergo a process of decay and gradually transform into a new elements (daughter nuclei).
Radioactivity is an inherent property and natural characteristic of unstable radioactive elements. Radioactivity is an irreversible process and occurs continuously all the time.
As a result of emission of radiation the atoms of these elements undergo a process of decay and gradually transform into a new elements (daughter nuclei).
Radioactivity is an inherent property and natural characteristic of unstable radioactive elements. Radioactivity is an irreversible process and occurs continuously all the time.
Ionizing and Non-ionizing Radiation
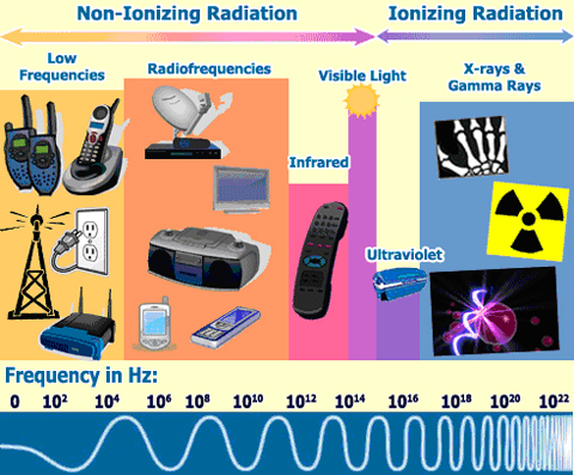
Radiation is distinguished in two main categories: ionizing and non-ionizing radiation.
Radiation is distinguished in two main categories: ionizing and non-ionizing radiation.
- Ionizing radiation is of high energy, capable of penetrating matter, to produce ionization of the atoms, to break chemical bonds and cause harm in living matter. These include X-rays and γ-rays
- Non-ionizing radiation is of lower energy, not capable of causing ionization.Non-ionizing radiation ranges from extremely low frequency radiation, shown on the far left through the audible, microwave, and visible portions of the spectrum into the ultraviolet range.
Background radiation
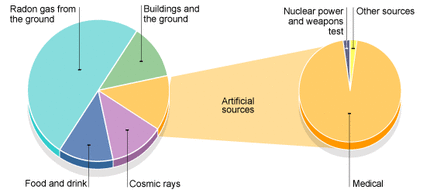
Background radiation is all around us. Some of it comes from natural sources and some comes from artificial sources.
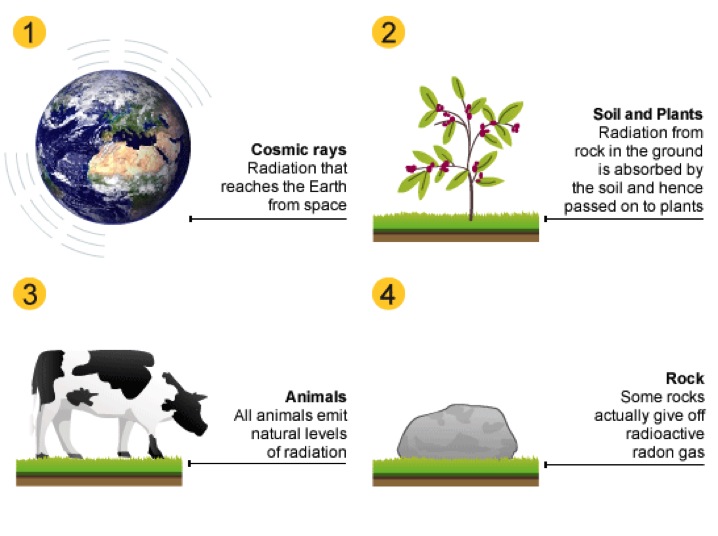
Natural sources of background radiation include:
Background radiation is all around us. Some of it comes from natural sources and some comes from artificial sources.
Natural sources of background radiation include:
- Cosmic rays - radiation that reaches the Earth from space
- Rocks and soil - some rocks are radioactive and give off radioactive radon gas
- Living things - plants absorb radioactive materials from the soil and these pass up the food chain
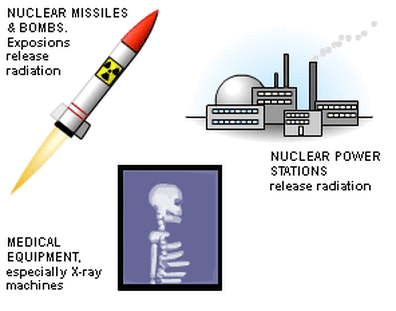
Artificial sources of background radiation include:
There is little we can do about natural background radiation. After all, we cannot stop eating, drinking or breathing to avoid it!
However, human activity has added to background radiation by creating and using artificial sources of radiation. These include radioactive waste from nuclear power stations, radioactive fallout from nuclear weapons testing and medical x-rays.
There is little we can do about natural background radiation. After all, we cannot stop eating, drinking or breathing to avoid it!
However, human activity has added to background radiation by creating and using artificial sources of radiation. These include radioactive waste from nuclear power stations, radioactive fallout from nuclear weapons testing and medical x-rays.
15.1 Detection of radioactivity
Photographic film
|
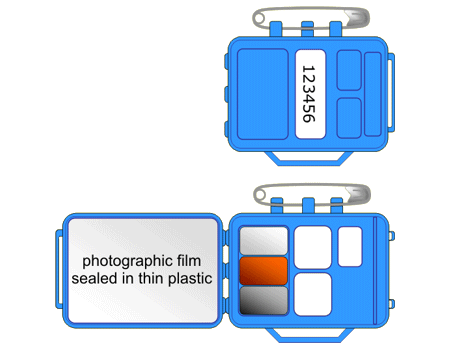
Photographic film goes darker when it absorbs radiation, just like it does when it absorbs visible light. The more radiation the film absorbs, the darker it is when it is developed.
People who work with radiation wear film badges, which are checked regularly to monitor the levels of radiation absorbed. The diagram shows a typical radiation badge when it is closed and what the inside looks like when it is opened. |
|
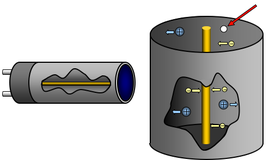
The Geiger-Müller (G-M) tube
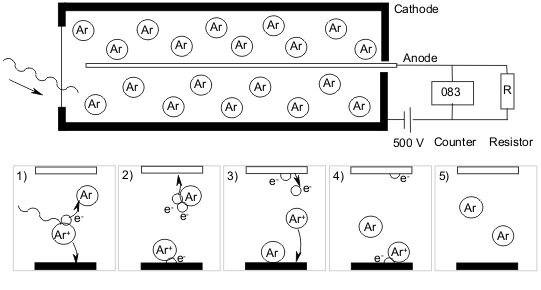
As ionizing radiation coming from the surrounding medium passes through the mica window and enters the Geiger-Muller tube, it ionizes the gas inside, transforming it into positively charged ions and electrons. The electrons eventually migrate towards the anode of the tube detector, while the positively charged ions accelerate towards the cathode. As the positive ions move towards the cathode, they collide with the remaining inert gas thus producing more ions through an avalanche effect. When this happens an electrical current is established between the two electrodes.
|
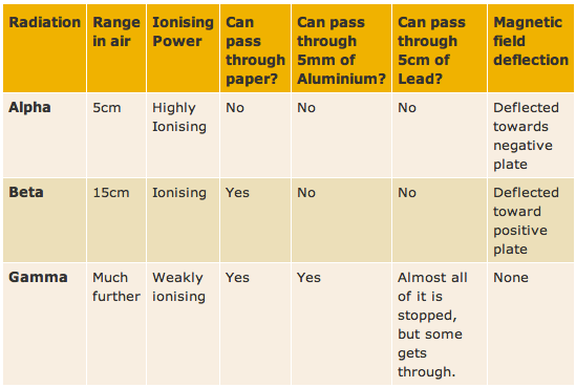
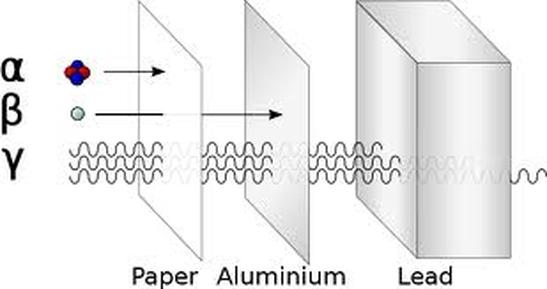
15.2 Characteristics of the three kinds of emission
|
There are 3 types of ionising radiation:
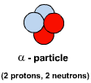
ALPHA - α - is a helium nucleus, 2 protons and 2 neutrons. It has mass of 4 a.m.u. and a strong positive charge of +2 BETA - β- is a fast moving electron. It has mass (small) and a negative charge of -1 GAMMA - γ - is a high-energy electromagnetic waves. Gamma rays are caused by changes within the nucleus. They are part of the Electromagnetic Spectrum and so travel at the speed of light. They have no mass and no charge. |
15.3 Radioactive decay
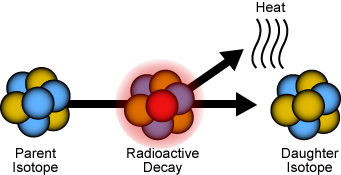
Radioactive decay The nuclei of some isotopes are unstable. They can split up or ‘decay’ and release radiation. Such isotopes are called radioactive isotopes or radioisotopes. When a radioactive isotope decays, it forms a different atom with a different number of protons.
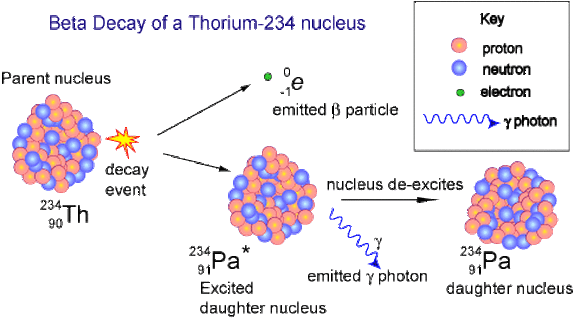
When an atom emits alpha or beta radiation, its nucleus changes. It becomes the nucleus of a different element. This is because the number of protons in the nucleus determines which element the atom belongs to. These are the changes that occur to the number of particles in an unstable nucleus when it emits a radioactive particle.
When an atom emits alpha or beta radiation, its nucleus changes. It becomes the nucleus of a different element. This is because the number of protons in the nucleus determines which element the atom belongs to. These are the changes that occur to the number of particles in an unstable nucleus when it emits a radioactive particle.
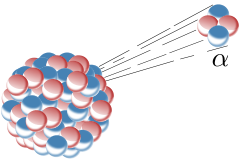
Alpha decay happens to reduce the number of nucleons in the nucleus, usually because it is so big that the Strong Nuclear force does not extend across sufficient a range to hold the protons in the nucleus together, and they are repelled electrostatically.
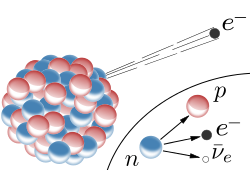
Beta decay usually happens to redress the balance between neutrons and protons in the nucleus. In beta decay at this level we consider it to be a neutron becoming a proton and emitting an electron.
NB. In all nuclear reactions charge must be conserved.
NB. In all nuclear reactions charge must be conserved.
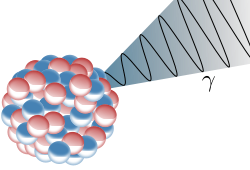
Gamma decay usually accompanies one of the previous types of decay as it is simply the emission of energy. Thus allowing energy/mass conservation to apply. Sometimes, after its emission of an alpha, beta or positron particle, the nucleus is still in an excited state, called a metastable state (m). In order to get to a lower energy state it emits a quantum of energy in the form of a gamma ray
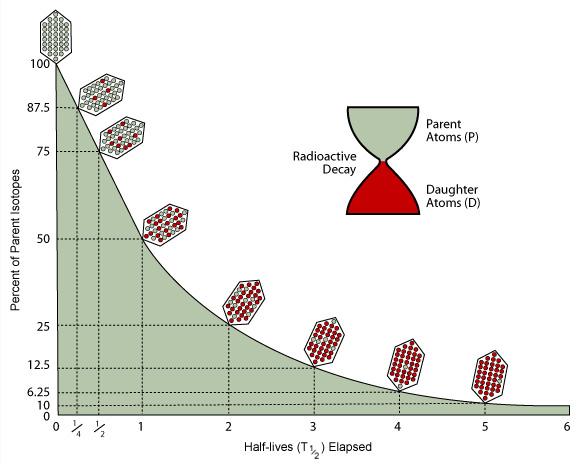
15.4 Half-life
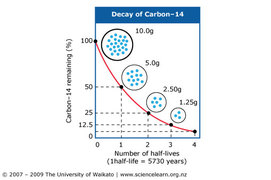
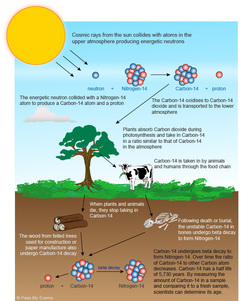
The nuclei of radioactive atoms are unstable. They break down and change into a completely different type of atom. This is called radioactive decay. For example, carbon-14 decays to nitrogen-14 when it emits beta radiation.
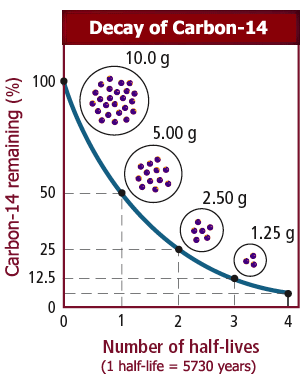
It is not possible to predict when an individual atom might decay. But it is possible to measure how long it takes for half the nuclei of a piece of radioactive material to decay. This is called the half-life of the radioactive isotope.
Two definitions
There are two definitions of half-life, but they mean essentially the same thing:
Graphs
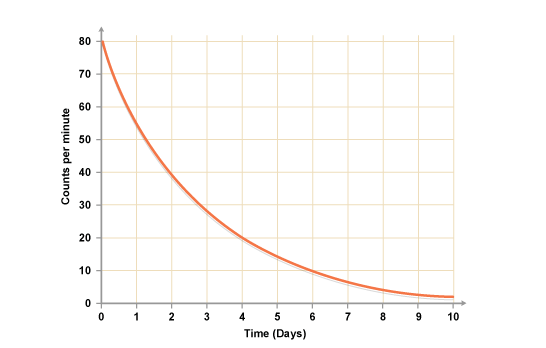
It is possible to find out the half-life of a radioactive substance from a graph of the count rate against time. The graph shows the decay curve for a radioactive substance.
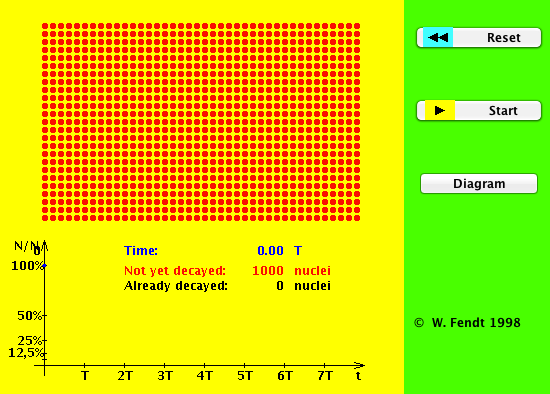
Radioactive Decay: A Sweet Simulation of Half-Life
It is not possible to predict when an individual atom might decay. But it is possible to measure how long it takes for half the nuclei of a piece of radioactive material to decay. This is called the half-life of the radioactive isotope.
Two definitions
There are two definitions of half-life, but they mean essentially the same thing:
- the time it takes for the number of nuclei of the isotope in a sample to halve
- the time it takes for the count rate from a sample containing the isotope to fall to half its starting level
Graphs
It is possible to find out the half-life of a radioactive substance from a graph of the count rate against time. The graph shows the decay curve for a radioactive substance.
Radioactive Decay: A Sweet Simulation of Half-Life
15.5 Safety precautions
|
You cannot do much to reduce your exposure to natural background radiation, but great care is needed when handling radioactive materials.
Precautions include:
|
15.6 The nuclear atom – Isotopes
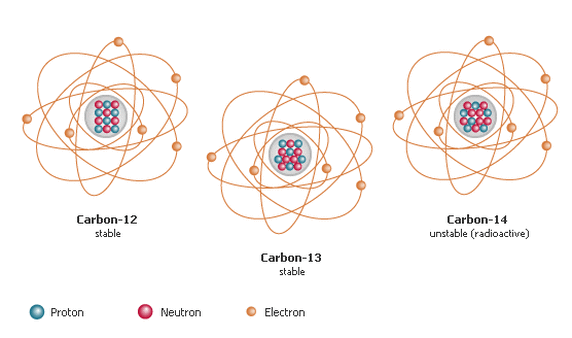
Isotopes are atoms of an element with the normal number of protons and electrons, but different numbers of neutrons. Isotopes have the same atomicnumber, but different mass numbers.
The different isotopes of an element have identical chemical properties. However, some isotopes are radioactive. These are known as radioisotopes.
The different isotopes of an element have identical chemical properties. However, some isotopes are radioactive. These are known as radioisotopes.
What are nuclides and radionuclides?
Nuclide is a term used to categorize different forms of atoms very specifically. Each nuclide has a unique set of characteristics
Nuclide is a term used to categorize different forms of atoms very specifically. Each nuclide has a unique set of characteristics
- number of protons
- number of neutrons
- energy state.
Uses of radiation- Ionisation
Nuclear radiation ionises materials. This changes atoms or molecules into charged particles.
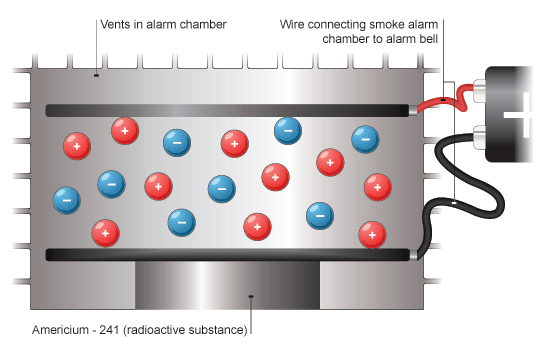
Smoke Detectors
Smoke detectors alert people to fires. Smoke from the fire is detected by the device, which then gives off an alarm. One type of smoke detector uses americium-241, a source of alpha radiation, to detect smoke. The alpha radiation ionises the air particles inside the smoke detector. This allows a small electric current to flow. If there is a fire, smoke particles going into the detector are hit by alpha radiation. This reduces the ionisation of the air particles causing the current to drop. The drop in current is detected by the smoke detector, setting off the alarm.
Tracers
Radioisotopes are used as tracers in industry. These are used for tracking substances. For example, they can be used to:
Radioisotopes are used as tracers in industry. These are used for tracking substances. For example, they can be used to:
- Find leaks or blockages in underground pipes
- Find the route of underground pipes
- Track the dispersal of waste
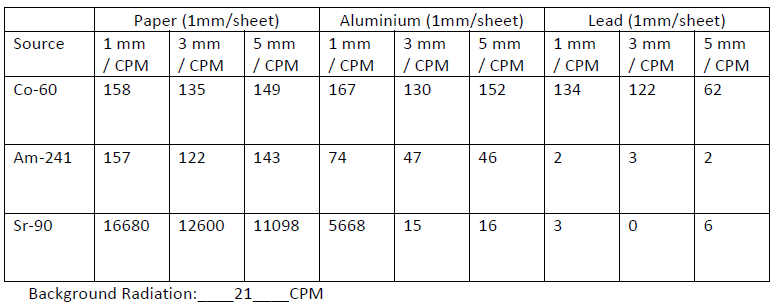
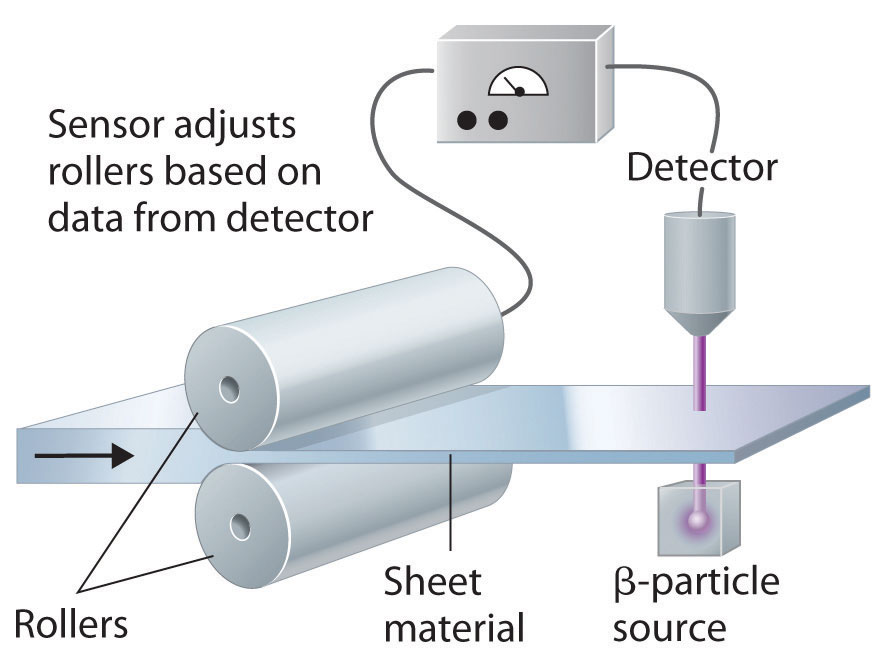
Using Radiation to Control the Thickness of a Material
Radiation is used in industry in detectors that monitor and control the thickness of materials such as paper, plastic and aluminium. The thicker the material, the more radiation is absorbed and the less radiation reaches the detector. It then sends signals to the equipment that adjusts the thickness of the material. Check your understanding of this by watching the simulation.
Radiation is used in industry in detectors that monitor and control the thickness of materials such as paper, plastic and aluminium. The thicker the material, the more radiation is absorbed and the less radiation reaches the detector. It then sends signals to the equipment that adjusts the thickness of the material. Check your understanding of this by watching the simulation.
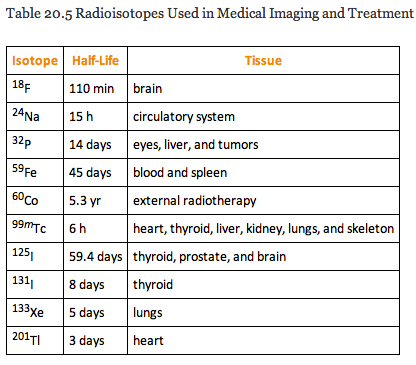
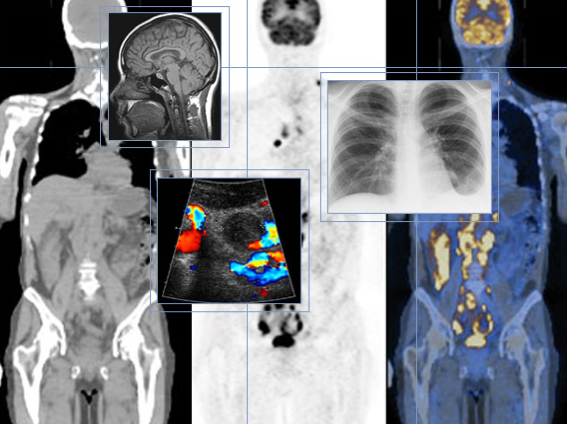
Medical Imaging and Treatment with Radioisotopes
Doctors may use radioactive chemicals called tracers for medical imaging. Certain chemicals concentrate in different damaged or diseased parts of the body, and the radiation concentrates with it. Radiation detectors placed outside the body detect the radiation emitted and, with the aid of computers, build up an image of the inside of the body.
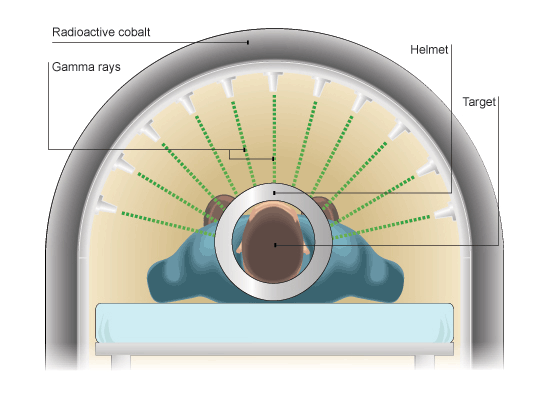
Gamma rays damage cells whether they are normal or cancerous, so gamma rays must be focused on the tumour. One way of doing this is to use a wide beam of gamma rays, but to rotate the beam around the patient, keeping the tumour at the centre. This concentrates the gamma rays on the cells that need to be killed.
Radioactive dating
Because the radioactive half-life of a given radioisotope is not affected by temperature, physical or chemical state, or any other influence of the environment outside the nucleus save direct particle interactions with the nucleus, then radioactive samples continue to decay at a predictable rate. That is, any radioactive nucleus acts as a clock. If determinations or reasonable estimates of the original composition of a radioactive sample can be made, then the amounts of the radioisotopes present can provide a measurement of the time elapsed.
Because the radioactive half-life of a given radioisotope is not affected by temperature, physical or chemical state, or any other influence of the environment outside the nucleus save direct particle interactions with the nucleus, then radioactive samples continue to decay at a predictable rate. That is, any radioactive nucleus acts as a clock. If determinations or reasonable estimates of the original composition of a radioactive sample can be made, then the amounts of the radioisotopes present can provide a measurement of the time elapsed.